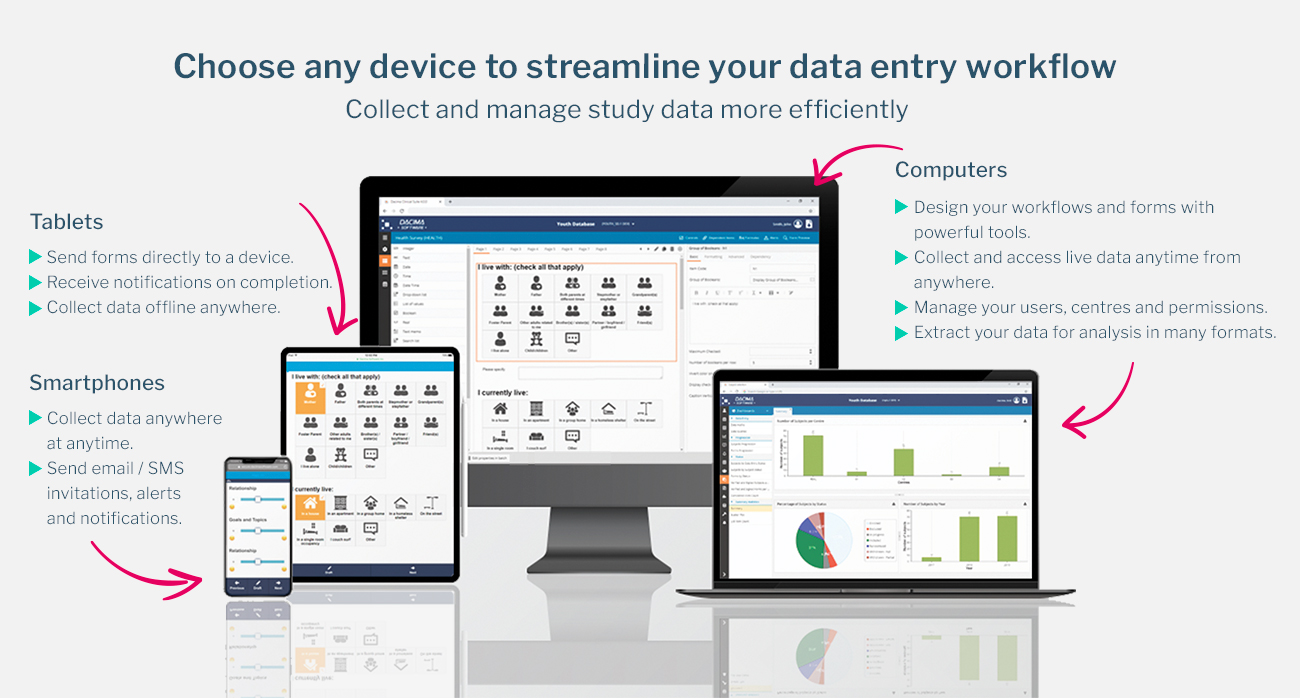
In today’s rapidly evolving world of healthcare and clinical research, technology continues to reshape how data is gathered, analyzed, and used to improve patient outcomes. One of the most significant advancements in this space is the integration of Electronic Patient Reported Outcomes, often referred to as ePRO. This digital approach to patient feedback collection has transformed how researchers, clinicians, and regulators understand the real-world impact of treatments and interventions.
At its core, ePRO refers to the process where patients directly report their health status, symptoms, treatment experiences, and overall quality of life using electronic devices such as tablets, smartphones, or web-based applications. Unlike traditional paper-based methods, ePRO ensures accuracy, timeliness, and reliability in data capture, reducing the risk of lost or incomplete information.
Why Electronic Patient Feedback Matters
Understanding the patient’s perspective is critical to evaluating the true effectiveness of any medical treatment. Patients experience side effects, improvements, and changes in their day-to-day lives that may not always be visible through laboratory results or physician assessments alone. By allowing patients to record their own health experiences in real time, electronic reporting tools provide researchers with an authentic view of how treatments perform in real-world settings.
Moreover, this patient-centered approach enhances clinical trial transparency and patient engagement. When individuals feel their input directly contributes to medical progress, they are more likely to stay motivated and compliant throughout the study duration. This not only improves data completeness but also strengthens the validity of study outcomes.
Key Advantages of Using Electronic Tools for Patient Outcomes
The transition from traditional paper questionnaires to electronic systems brings a host of advantages that are now indispensable in modern clinical trials:
-
Accuracy and Data Integrity – Digital systems minimize transcription errors and incomplete fields. Built-in logic checks prevent contradictory responses or skipped questions, ensuring higher-quality data.
-
Real-Time Data Capture – Patients can log their experiences as they happen, rather than waiting for scheduled appointments. This real-time feedback enhances the precision of symptom tracking and allows for timely intervention if issues arise.
-
Improved Compliance – Automated reminders and user-friendly interfaces encourage patients to complete their reports consistently, reducing dropout rates and ensuring a more reliable data set.
-
Global Accessibility – With the increasing availability of mobile devices and internet access, clinical trials can now gather patient data from diverse geographical locations without logistical barriers.
-
Regulatory Confidence – Regulatory authorities such as the FDA and EMA recognise ePRO as a valid source of clinical evidence when evaluating new treatments. The ability to provide transparent, verifiable, and auditable data strengthens the overall credibility of a study.
The Role of Technology in Enhancing Patient Experience
Beyond data collection, electronic reporting tools are designed to simplify the process for patients. The best systems are intuitive, accessible to individuals of varying technical skills, and available in multiple languages. Patients can complete assessments from the comfort of their homes, significantly reducing travel time and the burden of clinic visits.
Additionally, the digital format allows the integration of multimedia features—such as audio instructions or symptom illustrations—that make the process more interactive and easier to understand. These innovations are particularly valuable for older adults or patients with limited literacy, ensuring inclusivity in research participation.
Furthermore, digital systems can automatically adapt to patient responses. For instance, if a participant reports severe side effects, the platform can trigger follow-up questions or alert study staff to initiate immediate medical review. This not only safeguards participant safety but also demonstrates how technology can act as a bridge between patients and clinical teams.
Data Security and Privacy Considerations
With the shift to electronic systems, ensuring data protection and patient confidentiality is a top priority. Reputable ePRO platforms incorporate secure encryption protocols, role-based access, and compliance with international standards such as GDPR and HIPAA.
Patients’ trust in the research process depends on knowing their information is protected. Transparent communication about how data is used, stored, and shared helps reinforce this trust. Ethical guidelines also require that patient consent be properly obtained before data collection begins, further strengthening accountability within clinical studies.
How ePRO Benefits Researchers and Sponsors
From a research management perspective, electronic systems streamline workflows, reduce operational costs, and accelerate study timelines. Because data is captured and transmitted instantly, researchers can monitor patient responses in real time, identify trends earlier, and make necessary adjustments without waiting for manual data entry or collation.
The availability of automated dashboards and analytical tools allows study sponsors and investigators to visualize patterns, track compliance rates, and assess outcomes dynamically. This digital insight not only increases efficiency but also enhances decision-making during both the trial and post-marketing surveillance phases.
Another critical benefit is data standardization. When patient feedback is captured through structured digital formats, it becomes easier to compare results across studies, populations, and treatment categories. This facilitates meta-analyses and contributes to broader scientific understanding.
Challenges in Implementing Electronic Reporting Systems
While the benefits are numerous, adopting electronic reporting tools also presents challenges. Not all patients are comfortable with technology, especially older populations or those living in regions with limited internet access. To overcome this, training and technical support should be integrated into study design to ensure inclusivity.
Cost can also be a concern for smaller research organizations, as initial setup and licensing fees may appear high. However, when weighed against the long-term savings from reduced manual labor, faster recruitment, and fewer data errors, the investment often proves worthwhile.
Additionally, ensuring interoperability between electronic reporting platforms and other clinical data management systems remains an area of continuous improvement. Seamless data integration is essential for maintaining efficiency and consistency across all study components.
Future Trends in Patient-Reported Data Collection
As technology continues to evolve, the next wave of innovation in patient-reported outcomes will likely include artificial intelligence and wearable devices. These advancements will allow researchers to combine subjective patient input with objective health data such as heart rate, sleep patterns, and physical activity levels.
Machine learning algorithms can analyze these combined datasets to predict treatment outcomes or detect early warning signs of adverse effects. Such predictive analytics will enable more personalized and proactive healthcare management, benefitting both patients and clinicians.
Moreover, the integration of voice recognition technology could further simplify reporting for patients who may struggle with typing or vision-related limitations. Multilingual voice input and real-time translation could make global clinical research even more inclusive.
The Broader Impact on Healthcare
The widespread use of electronic patient-reported systems goes beyond clinical trials—it is reshaping healthcare delivery as a whole. Hospitals and outpatient clinics are increasingly using these platforms to monitor chronic disease management, measure treatment satisfaction, and assess overall wellbeing.
By continuously capturing patient perspectives, healthcare providers gain actionable insights that help tailor care plans more effectively. This shift from episodic to continuous monitoring promotes preventive healthcare, reduces hospital readmissions, and enhances overall patient satisfaction.
Ultimately, technology-driven feedback systems empower patients to play an active role in their own health journey. When patients feel heard and involved, outcomes naturally improve.
The adoption of ePRO marks a pivotal advancement in the way patient data is collected and analyzed. By combining technology with patient engagement, this method delivers more accurate, efficient, and meaningful results that drive better healthcare decisions. As the global focus on patient-centered care continues to grow, ePRO will remain at the forefront of innovation in clinical research and beyond.
For organizations seeking reliable, secure, and efficient solutions to manage clinical data electronically, Dacima Software continues to contribute expertise and innovation in this evolving digital landscape.